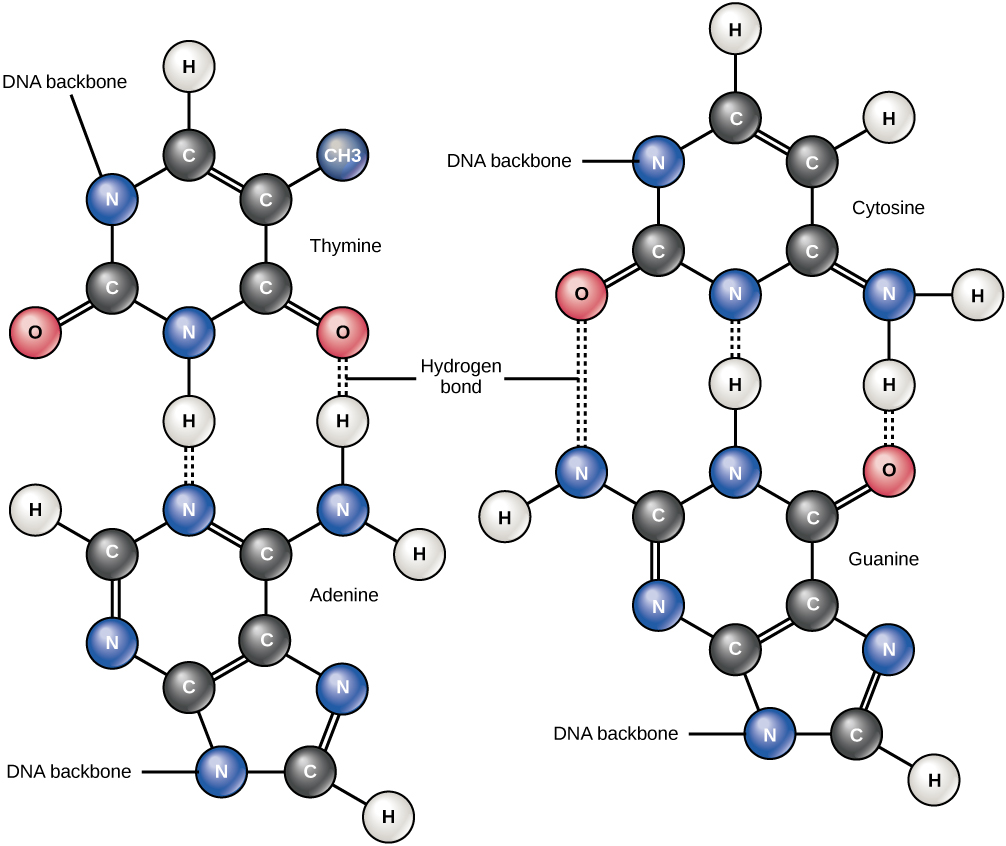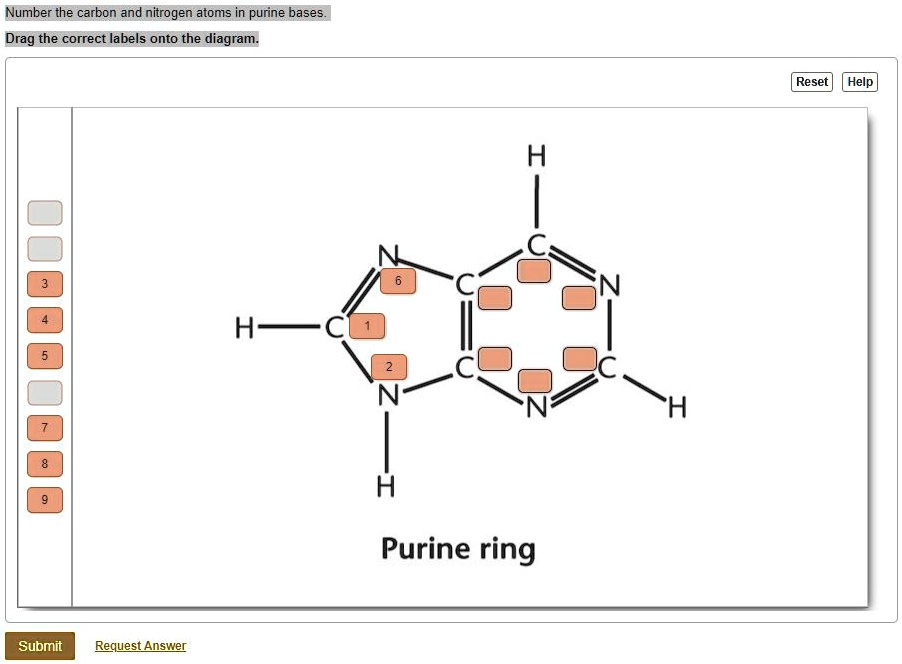
SOLVED: Number the carbon and nitrogen atoms in purine bases Drag the correct labels onto the diagram: Reset Help H H Purine ring Submit Request Answer
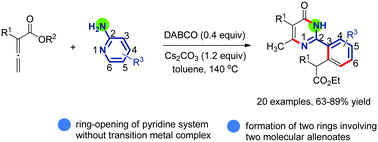
Carbon–nitrogen bond cleavage of pyridine with two molecular substituted allenoates: access to 2-arylpyrimidin-4(3H)-one - Chemical Communications (RSC Publishing)

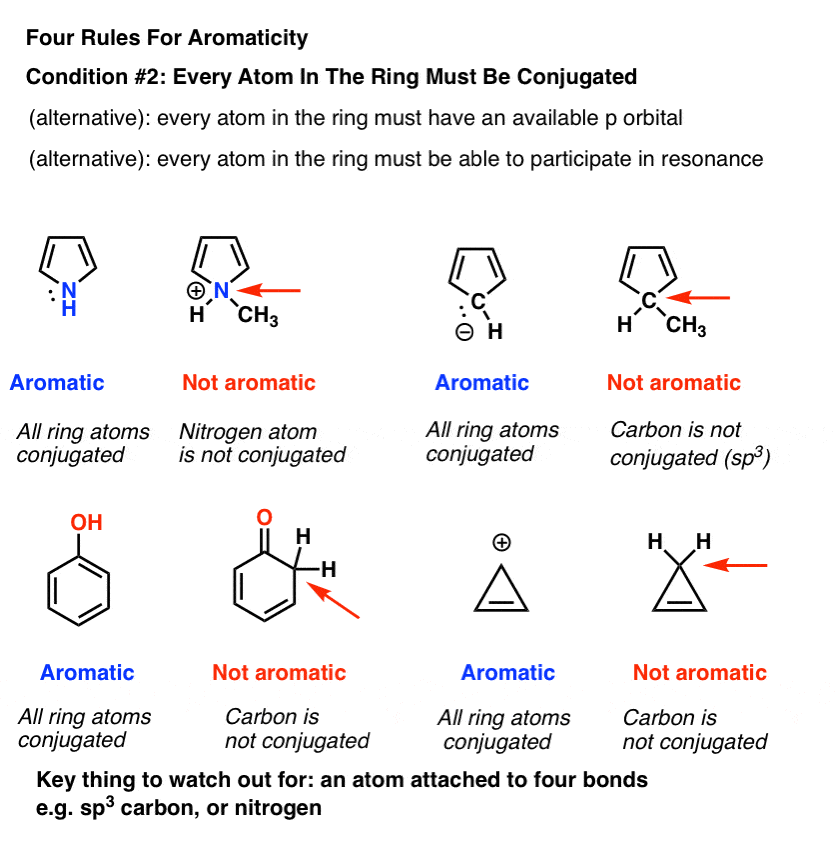
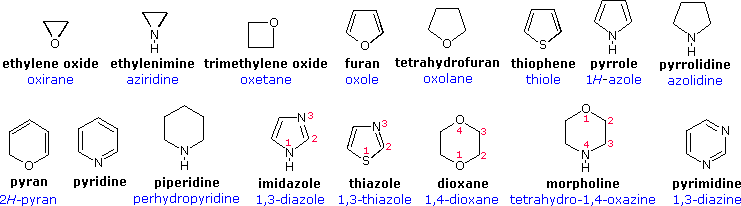
Nitrogen heterocycles are carbon organic rings containing nitrogen. Nitrogen heterocycles can be formed from aliphatic nitro… | Nitrogen, Amino acids, Organic rings
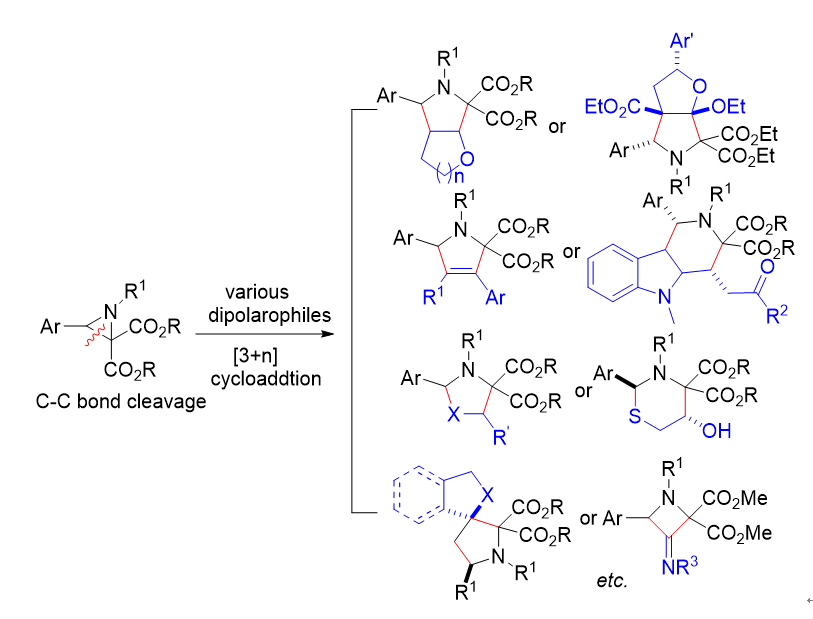
Recent Advances in Cycloaddition Reactions of Donor-Acceptor Aziridines via Carbon-Carbon Bond Cleavage

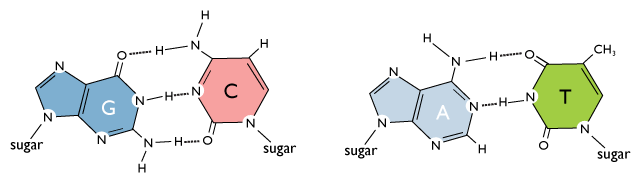
Carbon and nitrogen labelling of pyrimidine and purine rings. (a) When... | Download Scientific Diagram

Carbon–Hydrogen versus Nitrogen–Oxygen Bond Activation in Reactions of N-Oxide Derivatives of 2,2′-Bipyridine and 1,10-Phenanthroline with a Dimethylplatinum(II) Complex | Organometallics

Carbon–Carbon Bonding between Nitrogen Heterocyclic Carbenes and CO2 | The Journal of Physical Chemistry A


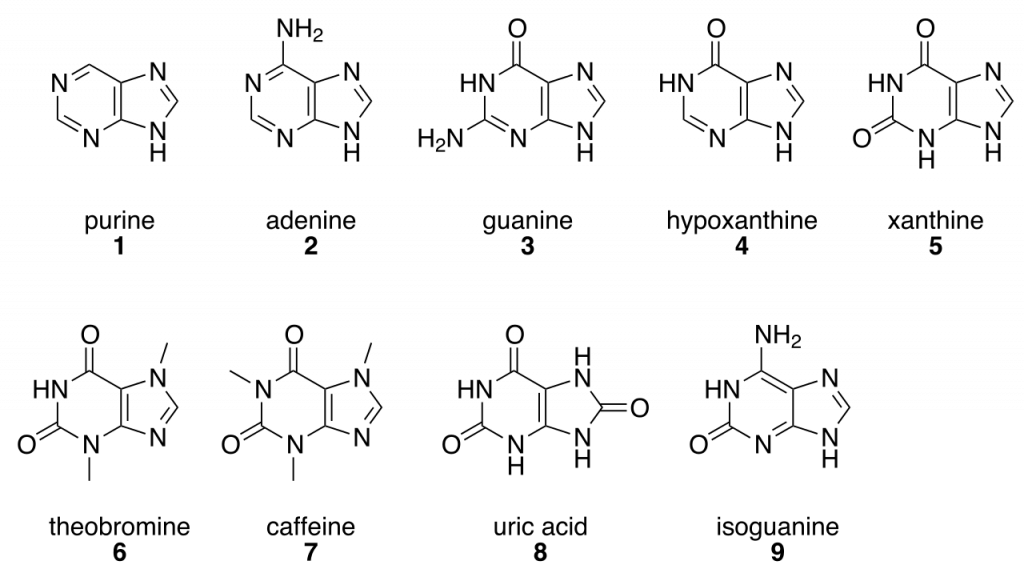
:max_bytes(150000):strip_icc()/purine-and-pyrimidine-nitrogenous-bases---skeletal-chemical-formulas-475632152-d34de0fec4e14f108a3e32901a1386c8.jpg)